Wish to use our Mai Device in your practice? Contact us today!
Mai Medical is a Medical Artificial intelligence platform that uses cutting-edge medical technology and holistic healthcare collaboration to comprehensively improve medical care.
The Need
85% of post-surgical patients
experience moderate to severe pain, impacting recovery and quality of life
1 in 5 people worldwide
suffer from chronic pain, making it a leading cause of disability
150,000 people die annually
from drug overdoses, with opioid pain medications being the leading cause
What is the MAI Device?
A Revolutionary Solution to Pain Relief
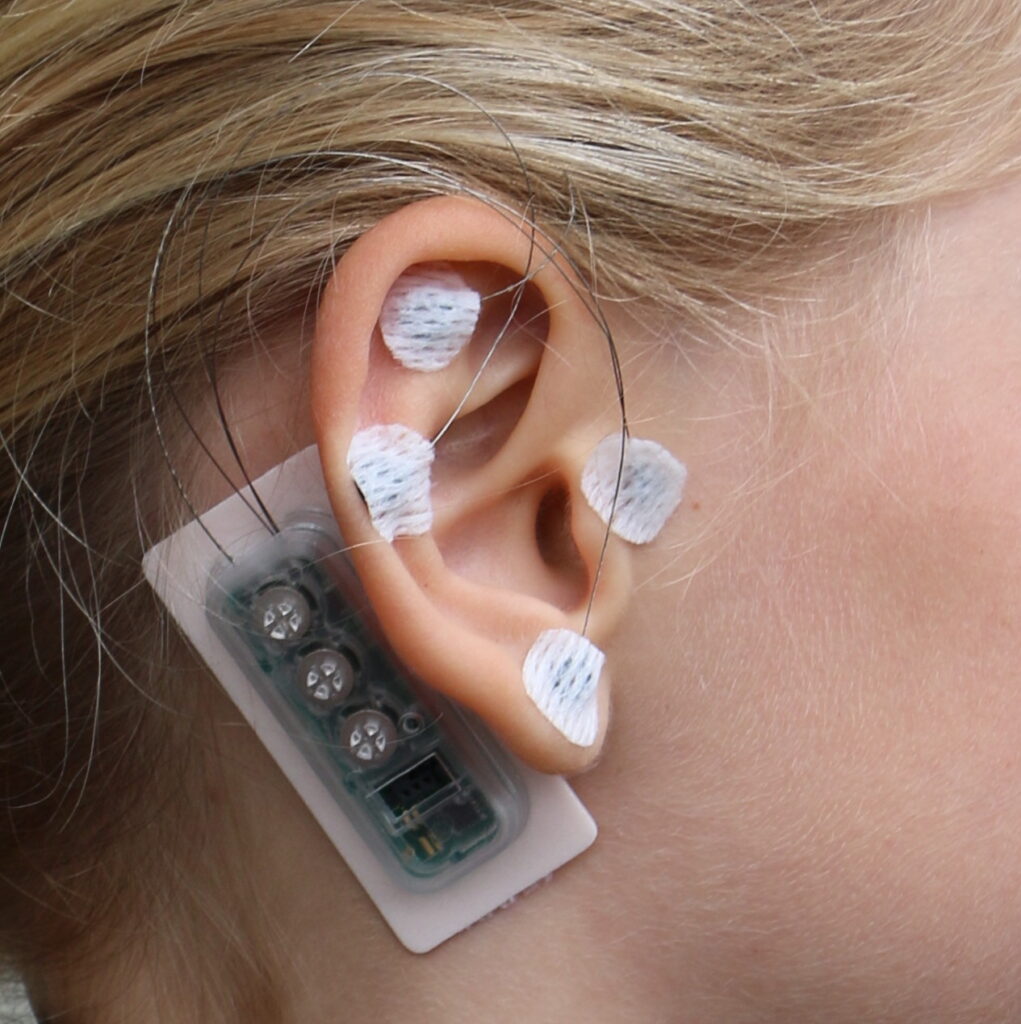
The MAI Device is a wearable device developed to effectively treat pain and other unpleasant symptoms through safe neuromodulation – without any surgery or medications. It is an auricular percutaneous electrical nerve field stimulator – a medical device that gently stimulates nerves in the ear that are connected to brain pathways responsible for the feeling of pain.
The MAI Device strives to safely provide substantial pain relief, thereby helping patients reduce medications, avoid surgery, and return to an active lifestyle.

Features
Non-surgical and Minimally Invasive
No cutting, implants, or permanent body changes.
Non-pharmacological and Non-addictive
No medications or chemicals involved.
Simple Application
Applied by a trained healthcare professional in any medical setting, without anesthesia or hospital-stay.
Discreet, Easy to wear
Does not interfere with daily life or activities - just avoid getting it wet.
TECHNOLOGY
How does it work?
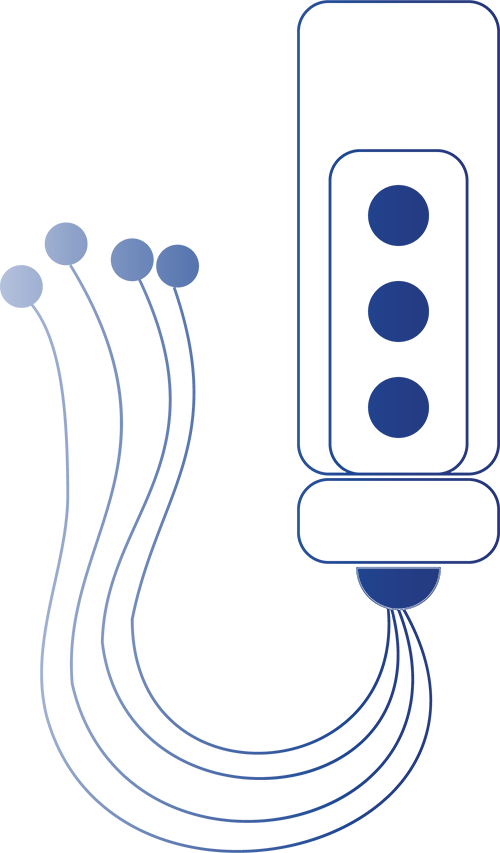
The MAI Device is worn on one ear for just 3 to 7 days and then removed. Four 2mm micro- needles are placed on the ear and stimulate specific cranial and occipital nerves, which are directly connected to brain pathways that process pain.
This targeted electrical stimulation interrupts pain signals and helps activate the body’s natural pain relief systems, including modulation of the parasympathetic nervous system.
Watch now: MAI Device Introduction Video
Outcomes
Rapid relief
Many patients feel a reduction in pain within minutes to hours of device placement.
Long-lasting Benefits
Relief may continue well after the device is removed.
Improved Well-being
Patients often report better sleep, mood, and mobility.
Reduced medication use
May reduce or eliminate the need for pain medications.
Comfortable use
The device is pain-free to wear, with only a quick, minor pinch during application.
Safety
The MAI Device is clinically tested and has shown no major risks or serious side effects in multiple studies and real-world applications. Side effects are rare, typically minor, and resolve on their own — such as light skin irritation or mild, temporary dizziness. It is considered safe even for patients with multiple comorbidities who may not be candidates for surgery.
The MAI Device has received FDA clearance for use in:
- Diabetic Neuropathy
- Post-surgical pain (e.g., after Cardiac surgery or C-section)
- Opioid Withdrawal Symptoms
- Irritable Bowel Syndrome (IBS) in children ages 11–18
While FDA clearance is limited, some studies on neuromodulation suggest it may have wider pain-relief potential. Ongoing clinical research continues to examine its broader potential applications. Speak with your healthcare provider to see if the MAI Device is right for you. FDA-clearance does not imply endorsement or effectiveness by the FDA. Individual results may vary.
The FDA (United States Food and Drug Administration) is an internationally respected and highly rigorous regulatory body, known for its strict evaluation process to ensure the safety and quality of medical devices.
Europe: the MAI Device is anticipated to complete EU certification and enter the market at the beginning of 2026.
Contraindications
The MAI Device should not be used by individuals who have:
- Cardiac pacemakers or defibrillators
- Spinal cord stimulators or other internal electric stimulation devices
- Hemophilia or uncontrolled bleeding disorders
- Psoriasis Vulgaris
- Pregnancy (insufficient data to confirm safety)
What patients say about the MAI Device
The Mai device is a pain-free, minimally invasive, non-surgical, and non-addictive medical device that treats pain and improves quality of life. It is exceptionally successful in safely and quickly treating pain caused by a wide variety of medical conditions.
John Smith, Company
The Mai device is a pain-free, minimally invasive, non-surgical, and non-addictive medical device that treats pain and improves quality of life. It is exceptionally successful in safely and quickly treating pain caused by a wide variety of medical conditions.
John Smith, Company
The Mai device is a pain-free, minimally invasive, non-surgical, and non-addictive medical device that treats pain and improves quality of life. It is exceptionally successful in safely and quickly treating pain caused by a wide variety of medical conditions.
John Smith, Company
Interested to know more about our products? Contact Us!
Frequently Asked Questions
The MAI Device can be applied in any medical setting by a trained healthcare professional.
It involves the placement of four tiny (2-millimeter) needles that just barely enter the skin of the ear. It is less invasive than getting a routine blood test. Patients may feel a brief, minor pinch when the needles are first inserted, but otherwise experience no pain or discomfort while wearing the device. It does not require surgery, anesthesia or hospital-stay.
Yes, the device is designed to be unobtrusive, allowing you to go about your daily activities comfortably. Feel free to work, exercise, and fulfill your responsibilities normally without the device interfering, just be careful to avoid getting the device wet.
The MAI Device is minimally-invasive, drug-free, non-surgical and non-addictive. It is only worn for 3 to 7 days and has no long-term implantation or permanent bodily changes. It is remarkably safe and studies have shown no major risks or side effects. These qualities make the MAI Device a notable alternative to current pain treatments, especially for patients that do not want to undergo risky, invasive procedures or take copious amounts of medications.
Please consult your healthcare provider to check whether treatment with the MAI Device is available for you and to learn more about the service and payment options.
Our Team of Experts
We are MAI Medical — a team dedicated to integrating the best human practices with cutting-edge technology to create innovative solutions for a healthier, more mindful, and longer life.

Pascal Fiore
Chief Executive Officer (CEO)

Rok Zaloznik
Chief Technology Officer (CTO), President of the Board, AI Expert

Dr. Peter Atallah
CHIEF MEDICAL OFFICER (CMO)

Eid Atallah
US department

Dr. Hong Phan
Medical Doctor (MD), Medical AdvisOr

Christian Schumann
Chief Quality Officer (CQO)

Michael Totilo
Chief Financial Officer (CFO)

Patrick Matzinger
Member of the Board
Our Partners